Introduction
Microfractures are actually an evolution of the original technique developed by Pridie in the 1950s(1) and improved upon by Steadman in the 1980s(2). This technique has become increasingly popular over the years, to the point where it now constitutes the reference for cartilage repair and the technique against which other procedures are compared. With all its variants, it remains the most widely used technique for repairing focal chondral lesions(3).
Since joint cartilage is a tissue without innervation or vascularization in most of its structure, it lacks the intrinsic capacity to generate an adequate response to tissue damage causing alteration of its structure, with local bleeding. The creation of subchondral bone perforations causes active bleeding from the bone marrow and from the microvascularization that perforates the subchondral plate, with penetration into the calcified layer of the cartilage. Such bleeding gives rise to a clot ("superclot" according to Steadman) that is formed in an environment which from the mechanical perspective is very hostile to it, and which can cause it to easily become detached before being able to complete its function(4).
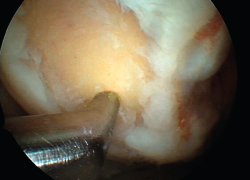
However, the microfractures technique in fact comprises a series of techniques grouped under the term of bone marrow stimulation (BMS). The techniques based on BMS have evolved over the years. Thus, the methodology has progressed from simple abrasion to large-calibre motorized perforations and non-motorized "ice pick" perforations (Figure 1), measuring between 1.5-2.5 mm in diameter, and finally to techniques that use small-calibre perforations with depths of up to 9 mm that are much less aggressive with the bone and marrow structure, and are referred to as subchondral drilling techniques.
[[{"fid":"4517","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"1":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"1"}}]]
Along with this technical evolution, a number of strategies have been added to cover and protect the blood clot as the basic component for starting the healing process, with a view to improving the outcomes. This is what is known as “microfracture plus”, enhanced BMS or also augmented microfracture(5,6).
The present article describes the biological principles on which these procedures are based, as well as some of the technologies that are used and which base their mechanism of action on principles we consider to be physiologically "elegant".
Biological principles
This series of techniques is based on biological principles, since the presence of the blood clot is essential for triggering the complex healing process in all human tissues. The mentioned blood clot contains all the elements needed to produce, in successive stages, the cascade of events - induction of the reparatory inflammatory response, followed by modulation of the latter and appearance of the new tissue through maturation of the granulation tissue - in a natural and coordinated manner, to finally give rise to the healing tissue. The latter may be identical to the original tissue (restitutio ad integrum), and in this case can technically be referred to as "regenerated tissue", or it may constitute a scar of variable quality depending on the degree to which it resembles the original tissue - in which case we speak of "repaired tissue".
In the immense majority of cases, joint cartilage repair results in healing tissue of variable quality depending on its hydration and the presence, orientation and percentage of type II collagen, which is characteristic of joint cartilage and is also referred to as hyaline cartilage. If this type of collagen is absent or is present in less than normal proportions, we speak of fibrocartilage. However, we prefer to use the term cartilage with more or less "hyaline characteristics", since this is closer to what is seen in the real life scenario. The more hyaline the cartilage obtained, i.e., the more stratified the tissue, with the presence of type II collagen in adequate amounts and locations, with correct hydration as evidenced by the presence of glycosaminoglycans (GAGs) and with adequate thickness, the better and more lasting the outcomes are expected to be. In contrast, when the presence of type I collagen increases versus the presence of type II collagen, with non-stratified or excessively or deficiently hydrated tissue, the outcome is a fibrous tissue (fibrocartilage) with inferior biomechanical performance, unable to perform its dual physiological function as a sliding surface and distributor of mechanical forces, and accelerated deterioration will thus result.
Since joint cartilage is a tissue without innervation or vascularization in most of its structure (but not in all of its structure, as we will see below), it lacks the intrinsic capacity to generate an adequate response to tissue damage causing a substantial alteration of its structural integrity, with local bleeding. The creation of subchondral bone perforations communicating the joint environment with the subchondral space causes active bleeding from the bone marrow and from the microvascularization that perforates the subchondral plate, with penetration into the calcified layer of the cartilage. Such bleeding gives rise to a clot ("superclot" according to Steadman) that is formed in an environment which from the mechanical perspective is very hostile to it, and which can cause it to easily become detached before being able to adequately complete its function.
The blood clot as primary factor
The blood clot has a number of functions, all of which are crucial, in the tissue healing process. However, damage to a vascularized tissue results in rupture of the local blood vessels. This in turn triggers a chain of perfectly coordinated events. Coagulation is the first event, and seeks to arrest bleeding as quickly as possible, since hemorrhage ultimately can threaten survival of the tissue.
The clot constitutes a biologically active three-dimensional barrier composed of fibrin and platelets. The platelets have two functions: a purely hemostatic function in which they accumulate at the walls of the ruptured vessel in an attempt to seal the perforation; and a secretory function upon becoming activated, characterized by immediate release of the elements contained in their "alpha" granules. In this way a number of cytokines are secreted, such as PDGF (platelet derived growth factor) and TGF-β (transforming growth factor beta), which contribute to induce an inflammatory response. In a second step, other similar substances such as VEGF (vascular endothelial growth factor) and FGF (fibroblast growth factor), among many others, modulate the response and the start of the repair activity (healing)(7).
Depending on the tissue involved, healing may be more or less sophisticated, ranging from simple and fibrous healing to structured repair with restitutio ad integrum of the damaged tissue - as occurs in the case of bone tissue.
Influences from subchondral bone
As its name indicates, subchondral bone is the zone underlying the joint cartilage, more specifically the calcified layer of the cartilage, with which it forms a "functional unit" with the rest of the cartilage(8). Subchondral bone constitutes the biomechanical transition between cartilage properly speaking - the role of which is to secure minimum friction and distribute the mechanical forces to which it is exposed - and the bone. The mechanical forces are absorbed by the subchondral bone and in turn are transmitted to the metaphysis and diaphysis of the bone.
The contact surface between cartilage and bone is not flat but rather wavy - thus affording a greater contact area and a very effective inter-blocking effect.
On the other hand, the subchondral region is highly vascularized, with arteriovenous microvascularization and a certain nerve supply. These structures penetrate through microcanals to reach the calcified layer of the cartilage. This has implications in the event of injury(9) and partly explains why full-thickness chondral lesions (corresponding to grade 4 of the International Cartilage Repair Society [ICRS] and Outerbridge score) are able to generate a spontaneous repair response, and also why BMS procedures (such as the microfractures technique) yield good (albeit not optimum) results. In addition, these features explain the need for curettage of the calcified layer of cartilage with these techniques, since its removal causes bleeding(10).
Influences from the synovial membrane
The chondrogenic process produced by the synovial membrane has received less attention than that associated with the subchondral bone. However, the mesenchymal cells from the synovial membrane have been shown to have a "tremendous chondrogenic potential"(11). In comparison with other sources such as the bone marrow or adipose tissue, these cells have shown to have a greater capacity to contribute to the chondrogenic process(12). This is not surprising, since the synovial membrane is well known for its role in maintaining joint homeostasis. The synovial membrane is richly innervated and vascularized - these being two important factors for producing a reparatory inflammatory response. For this reason, any implant placed over a focal cartilage lesion must be sufficiently porous to allow the penetration of chondrogenic cells and bioactive substances(13).
Microfracture plus
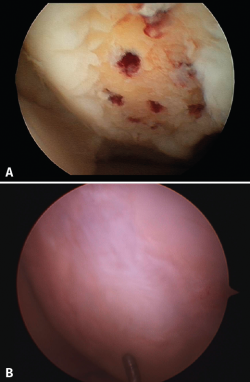
The results obtained with the microfracture techniques show variations, depending on the consulted literature source. This is due to the different hyaline characteristics of the regenerated cartilage. In some cases the percentage of fibrocartilage is high (Figure 2), due to a greater presence of type I collagen and the absence of type II collagen, in addition to the absence of a stratified cartilage tissue. Hyaline cartilage is characterized by the presence of type I collagen at the sliding surface, while type II collagen is the predominant form in the rest of the tissue, distributed to conform three basic stratification zones. These structural characteristics confer the biomechanical properties that allow long lasting function(13).
[[{"fid":"4518","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"2":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"2"}}]]
Experimental studies involving different microfracture augmentation techniques, as well as clinical studies, consistently report different degrees of improvement in the amount and quality (hyaline characteristics) of the new tissue, which should result in longer duration of the latter as well as afford improved clinical outcomes(14).
The basic biological principles are the same as those mentioned in reference to microfractures. Protection of the structure responsible for the blood clot is added, however, employing different natural biomaterials (collagens, alginates, fibrins, hyaluronic acid, hydroxyapatite, chitosan) and synthetic materials (polyglycolic and polylactic acids in their different variants), implanted in different formats (solid matrixes, membranes, gels). This contributes to the repair process.
As pointed out by Brittberg(15), one of the pioneers in chondrocyte culture technology, cells are always needed to repair tissue damage through biological means. What changes is the way in which they are used. One option is to attract the cells towards the lesion site by means of "something" that amplifies the physiological response - what Brittberg refers to as "intrinsic" use of the cells (in this case "in situ chondroinduction"), while another option is to grow the cells in culture and subsequently implant them ("extrinsic" use). Both options are valid, of course, though there may be indications where one is more appropriate than the other, based on aspects such as patient age, activity and height, as well as lesion depth or location. It is also necessary to consider aspects such as the cost-benefit ratio and surgeon experience when deciding to use one option or another.
This cellular substrate, which is not the topic of the present review, is referred to in several ways. The most common term is mesenchymal stem cells (MSCs)(16), renamed by Caplan(17) who initially described them in the late 1980s as medicinal signaling cells, since this term reflects their nature and action better. The group led by Caplan called for reflection upon the challenges posed by the chondrogenic differentiation of this cell line, particularly when believing that these cells alone are able to form hyaline cartilage(18). As orthopedic surgeons, our actions must favour and facilitate the functioning of this system, though in no case should we attempt to modify it, since this is where we can run into greater problems.
With the microfracture plus technique, the aim is to generate this cell population and the rest of the bioactive components that intervene in chondrogenesis, creating an optimum environment within the "synovial organ" (which is how synovial joints should be understood - with all which this term implies) in order for the process to develop as best as possible, and countering the negative influences derived from the hostile microenvironment which a joint represents.
Materials used
We do not intend to conduct an exhaustive review of the different material options and of some of the products currently available for improving the performance of microfractures. The key is to understand the rationale of the underlying mechanism of action. The choice of one technology or another depends on the surgeon. All the technologies based on these principles have something to offer when conceived on the basis of the described biological principles, with correct application of the latter. The classification that follows below is arbitrary.
AMIC membranes
The term "AMIC" refers to "autologous matrix-induced chondrogenesis". It was initially used in reference only to the utilization of a specific two-layer matrix of porcine origin composed of type I and type III collagen (Chondro-Gide®, Geistlich Biomaterials AG, Wolhusen-Switzerland)(19,20). In fact, AMIC® is a registered trademark of Geistlich, and the term theoretically should only be used in procedures that use this particular matrix. However, its use has also spread to other options, and we now speak of "AMIC-type" procedures. It has even been used concomitant to the implantation of autologous chondrocytes to seal the lesion and contain the chondrocytes in suspension(21).
As with all microfracture plus procedures, the chosen implant (in this case the membrane) seeks to protect the structural and functional integrity of the clot produced from BMS and, if possible, favour its action. In order to stabilize the membrane, the latter must be applied with fibrin glue at the margins in contact with the surrounding cartilage.
The most recent review related to this technique(22) analyses the results of 12 studies on focal chondral lesions of the knee. One of these studies involved a randomized, comparative design, with 375 patients. The study describes positive outcomes over the middle term (up to 5 years) in terms of both the clinical appearance and the structure of the new tissue. Sixty-six percent of the patients presented good repair quality as determined using semi-quantitative methods such as MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue). This technique has also been used in joints other than the knee, with positive results.
Other membranes can be found on the market, such as the widely used Hyalofast® (Anika Therapeutics, Padova, Italy), made from a hyaluronic acid ester base. More solid three-dimensional implants are also available, such as for example MaioRegen® (Finceramica, Faenza, Italy), made from type I collagen and hydroxyapatite in a stratified structure that is also useful for bone regeneration, as in patients with lesions characterized by an important osteochondral component. Another option is Agili-C™ (CartiHeal Ltd., Ariel, Israel), manufactured from aragonite (calcium carbonate derived from sea coral). At present, a randomized study is ongoing in the United States, comparing this product versus the standard of care, for presentation to the Food and Drug Administration (FDA) with the aim of obtaining its approval.
Gels and similar products
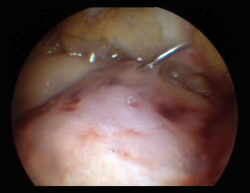
This section includes GelrinC® (Regentis Biomaterials, Akiva, Israel), based on a mixture of polyethylene glycol diacrylate (PEG-DA) and denaturalized fibrinogen, that reacts upon being exposed to ultraviolet light, with transformation into a semisolid gel. A comparative study with microfracture alone is also ongoing at this time in the United States, with a view to obtaining approval of the product. Another option among the hydrogels is CartiFill® (Sewon Cellontech, South Korea), based on atherocollagen, and which has been in use for some years in several countries (Figure 3).
[[{"fid":"4519","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"3":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"3"}}]]
One of the most widely known and studied materials, and to date the only material included in randomized clinical trials, is chitosan.
Chitosan
Chitosan is a natural polysaccharide derived from chitin; it is the second most abundant biological material in nature after cellulose. Chitosan is well known for its medical uses, in stimulating healing, and as antimicrobial and antihemorrhagic treatment, among other applications. It therefore was viewed as an attractive material for use in tissue engineering. It application in orthopedics is relatively recent, however.
The chemical structure of chitosan is very similar to that of the glycosaminoglycans (GAGs) (chondroitin sulfate and keratan sulfate), which are very important in hyaline cartilage structure and key elements for understanding its biomechanical behaviour. In addition, chitosan is able to interact with these substances and serve as a support for the production of aggrecan and type II collagen. This makes it a very attractive material for joint cartilage repair(23). On the other hand, chitosan adheres to the surface; no additional glue or adhesive is therefore needed.
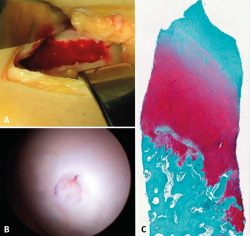
Despite these undeniable and proven benefits, however, chitosan is very unstable, and the elaboration of products containing it therefore poses major challenges. A very interesting property of this biopolymer is the fact that when in liquid state, it transforms into a semisolid gel upon reaching human body temperature(24). This discovery was very useful in searching for a formulation that may be injected into a joint such as the knee in the course of an arthroscopic procedure, with solidification in situ in only a few minutes. The decision was therefore made to use it as a coadjuvant in BMS procedures. In 2005, the Canadian company BioSyntech – posteriorly Piramal Healthcare (Canada), Bio-Orthopedics Division – started an international, randomized, comparative clinical study contrasting microfractures alone with microfracture plus versus their product, BST-CarGel® (currently Smith & Nephew, Wartford, UK). The results after both 12 months and at 5 years of follow-up evidenced sustained superiority over time of BST-CarGel® versus microfractures alone. This superiority was reflected by both the greater hyaline quality and quantity of the formed tissue, as measured objectively using quantitative magnetic resonance imaging (MRI) techniques(25,26) (Figure 4).
[[{"fid":"4520","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"4":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"4"}}]]
A posterior version of this technology, developed by the same team, appeared in 2013, in the form of JointRep® (Oligomedic Inc., Laval, Canada). This improved version makes use of a formulation with a firmer consistency, reaching gel state in under two minutes - thus facilitating arthroscopic implantation and shortening the surgery time. A comparative study on microfractures in 69 patients and involving a follow-up period of two years recorded substantial clinical improvement as measured by the WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) versus the control group(27). An international, randomized, comparative multicentre study in Australia, New Zealand and Canada was in the preparatory phase in late 2020.
Conclusions
A procedure as simple and widely used in medical practice over many years as the creation of a lesion zone to induce bleeding - as in BMS based procedures - can be complemented by tissue engineering tools with a view to favouring or even amplifying the biological response necessary for tissue repair in hyaline joint cartilage, and thus secure even better outcomes and in a more consistent manner. This is what is achieved with microfracture plus. Given the available options for improving the success rates with bone marrow stimulation "augmentation" processes, the regular use of some of such strategies is fully justified in order to improve the outcomes and maintain them for longer periods of time. This leads us to ask ourselves one final question: Based on the existing evidence, is the use of microfractures alone a desirable strategy?